
Historically, when estimates were done by hand: Sometimes the K values are nearly composition independent “hand” techniques of design/solution have used DePriester Charts (hydrocarbons):ħ DePriester Chart P = 2 bar T = 100 oC Isobutane others…. Historically, when estimates were done by hand: Seader & Henley (2006) Vapor phase: good liquid phase: good for HC or moderate – high pressures (compressible liquid phase) Typically used for liquid phase fugacity calculations Used for special cases (like gases dissolving into liquid phase) Typical simplifications: Ideal vapor phase Ideal liquid phase Raoult’s Law What are “Unit Operations” Brief thermodynamics review Binary flash with energy balance Multicomponent flash d.2 Overview Introduction UO course overview Equilibrium Stage separations Again, you are strongly encouraged to use a spreadsheet to do the calculations. Yi, V, and L for this flash distillation, if F = 1000 kmol/hr. You are encouraged to use a spreadsheet to do this calculation. Present your answer with a precision of 3 digits after the decimal place. b.) Double check your chart-reading for n-pentane using the McWilliams Equation (Eq. Include a screenshot or printout of your work.

a.) Determine K values for all of the components using the DePriester chart. You intend to flash a mixture at P = 30 psia and T = 140☏ that is 10 mol% propane, 20 mol% n-heptane, 30 mol% n-hexane, and the balance n-pentane. Note, if you wish to use the electronic chart, you will need to download and install Wolfram Player and then load the DePriesterChartForHydrocarbons.cdf.
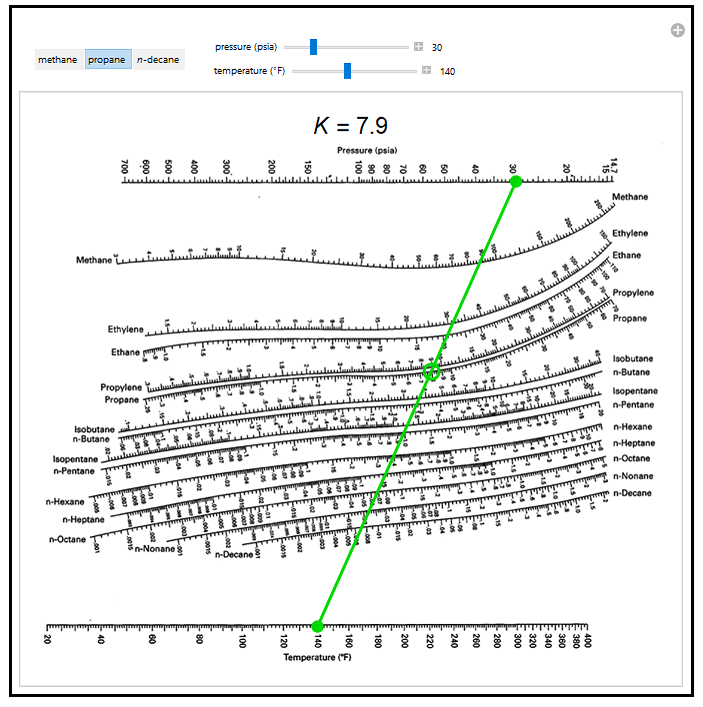
Transcribed image text: Use either the interactive UC Boulder Wolfram De Priester Chart or the chart in Wankat to answer the following questions.


 0 kommentar(er)
0 kommentar(er)
